Prior to Indian institute of science
J. Am. Chem. Soc. 2023, 145, 20053–20061.
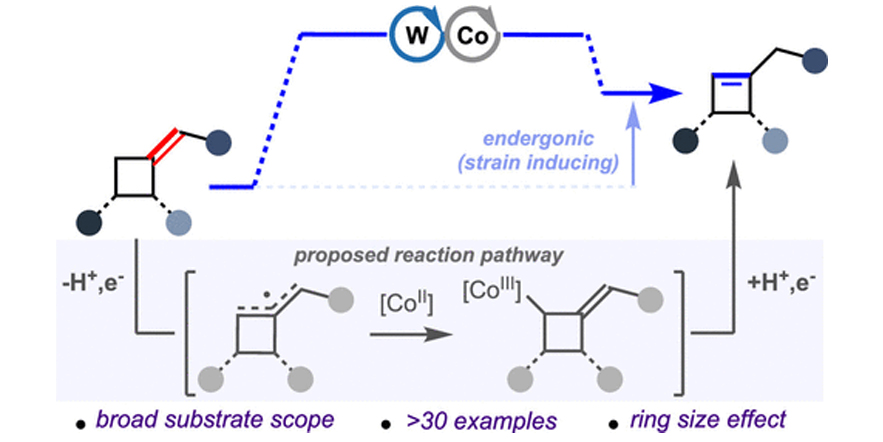
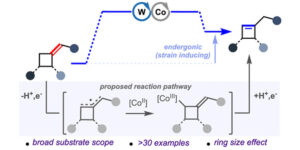
Strain-inducing positional alkene isomerization.
Palani, V.; Wendlandt, A. E
DOI: 10.1021/jacs.3c06935
Science 2022, 378, 383–390.
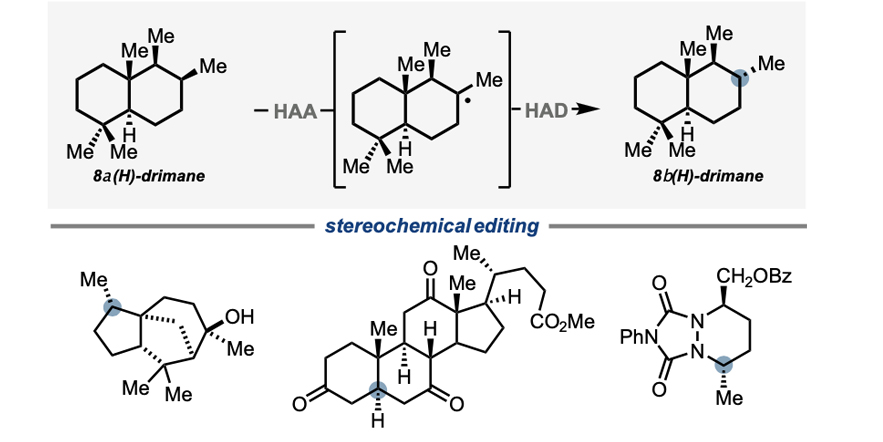
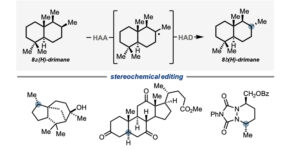
Stereochemical editing logic powered by the epimerization of unactivated tertiary stereocenters.
Zhang, Y.-A.*; Palani, V.*; Seim, A. E.; Wang, Y. Wang, K. J.; Wendlandt, A. E.
Highlighted in • C&E News • Chemistry World • In the pipeline
Nature 2022, 610, 40–41. (perspective)
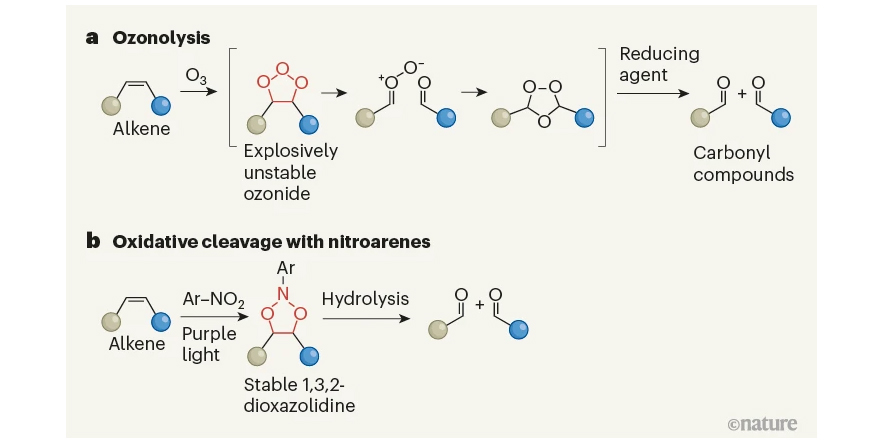
A stable alternative to an explosive synthetic reaction.
Palani, V.; Wendlandt, A. E.
DOI: 10.1021/jacs.3c06935
J. Am. Chem. Soc. 2022, 144, 145–152.
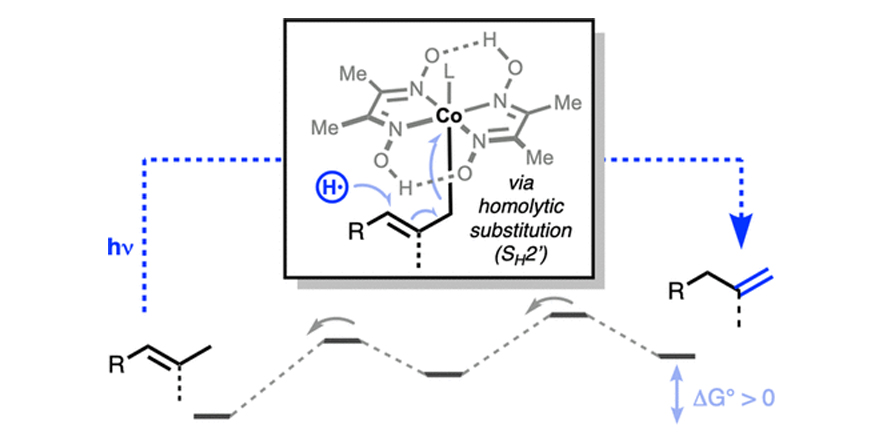
Catalytic, contra-thermodynamic positional alkene isomerization.
Occhialini, G.; Palani, V.; Wendlandt, A. E.
DOI: 10.1021/jacs.1c12043
Highlighted in • MIT News • Trends in Chemistry
Chem. Rev. 2022, 122, 10126–10169.
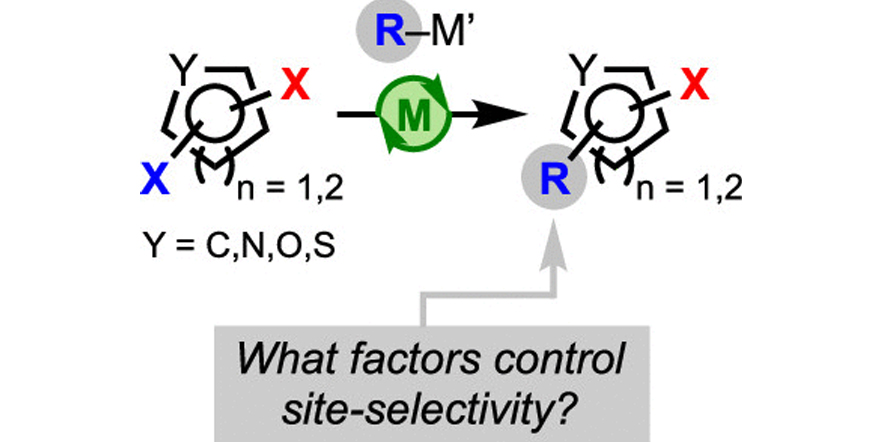
Site-selective cross-coupling of polyhalogenated arenes and heteroarenes with identical halogen groups.
Palani, V.*; Perea, M. A.*; Sarpong, R.
DOI: 10.1021/acs.chemrev.1c00513
Chem. Sci. 2021, 12, 1528–1534.
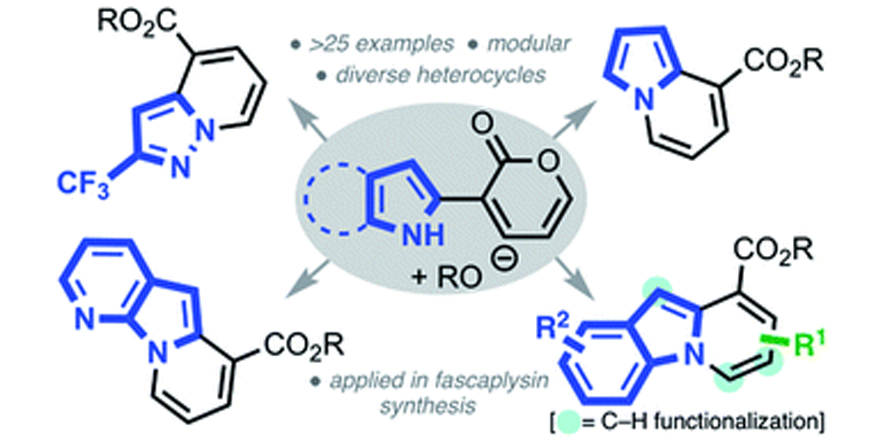
A pyrone remodeling strategy to access diverse heterocycles: application to the synthesis of fascaplysin natural products
Palani, V.; Perea, M. A.; Gardner, K. E.; Sarpong, R.
DOI: 10.1039/d0sc06317g
J. Am. Chem. Soc. 2019, 141, 14421–14432.
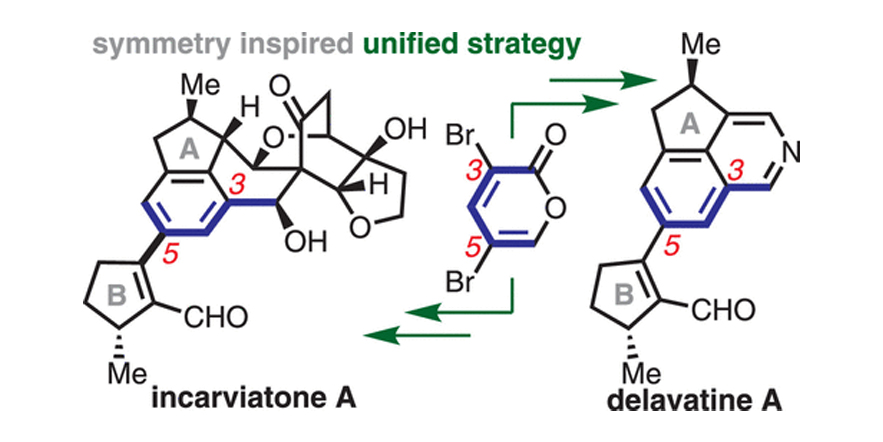
A Unified Strategy for the Enantiospecific Total Synthesis of Delavatine A and Formal Synthesis of Incarviaton A.
Palani, V.; Hugelshofer, C. L.; Sarpong, R.
DOI: 10.1021/jacs.9b07693
J. Am. Chem. Soc. 2019, 141, 2652–2660.
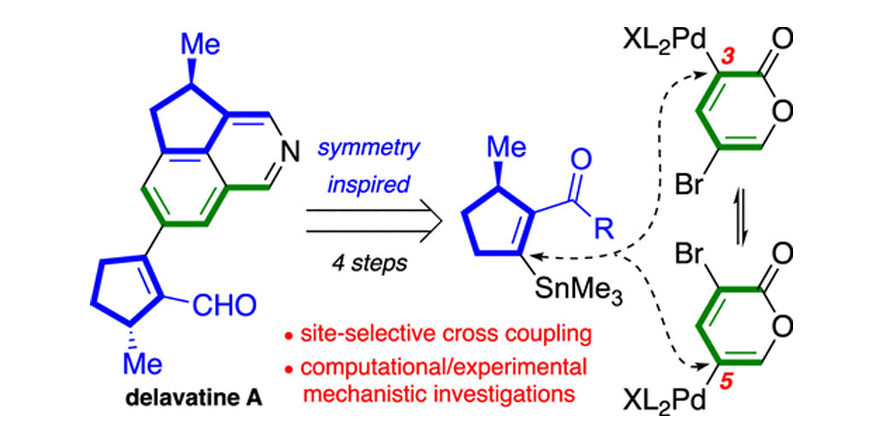
A Short Synthesis of Delavatine A Unveils New Insights into Site-Selective Cross-Coupling of 3,5-Dibromo-2-pyrone.
Palani, V.; Hugelshofer, C. L.; Kevlishvili, I.; Liu, P.; Sarpong, R.
DOI: 10.1021/jacs.8b13012
J. Am. Chem. Soc. 2019, 141, 8431–8435.
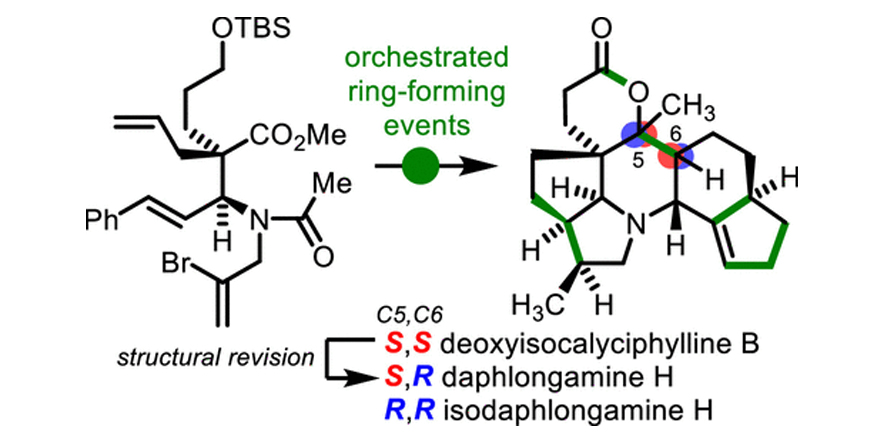
Calyciphylline B-type Alkaloids: Total Syntheses of (–)-Daphlongamine H and (–)-Isodaphlongamine H.
Hugelshofer, C. L.; Palani, V.; Sarpong, R.
DOI: 10.1021/jacs.9b03576
J. Org. Chem. 2019, 84, 14069–14091.
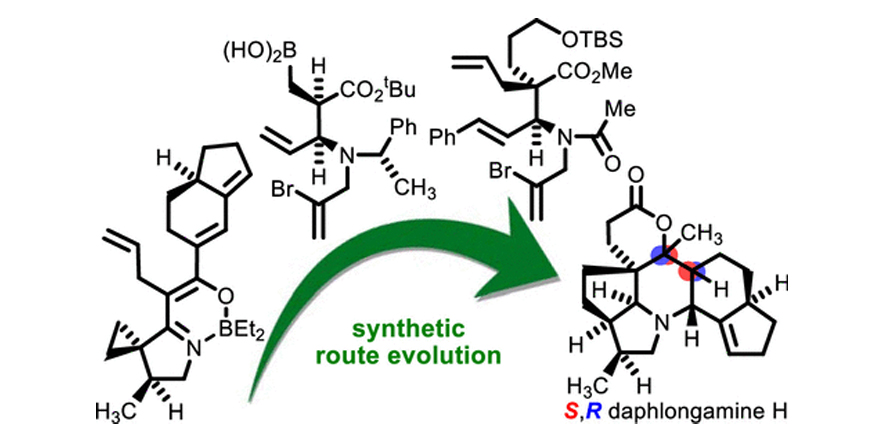
Calyciphylline B-type Alkaloids: Evolution of a Synthetic Strategy to (–)-Daphlongamine H.
Hugelshofer, C. L.; Palani, V.; Sarpong, R.
Org. Lett. 2018, 20, 2649–2653.
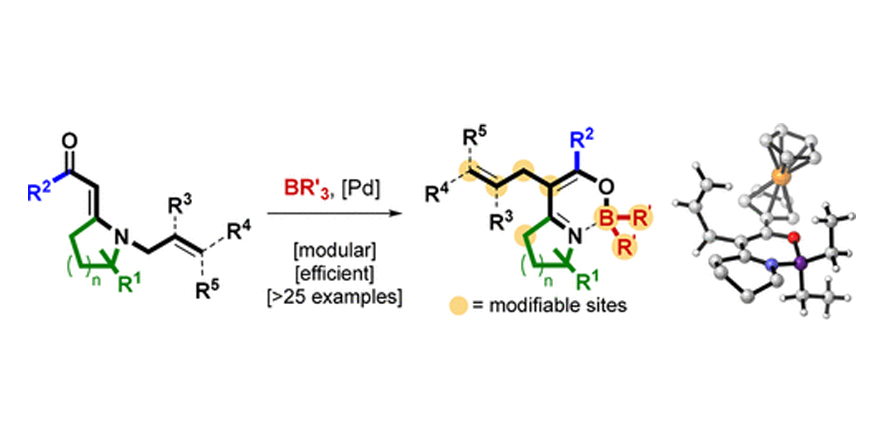
Oxazaborinines from Vinylogous N-Allylic Amides: Reactivities of Underexplored Heterocyclic Building Blocks.
Hugelshofer, C. L.; Palani, V.; Sarpong, R.
DOI: 10.1021/acs.orglett.8b00859
Org. Lett. 2018, 20, 8082–8085
Reactions of Diaziridines with Benzynes Give N-Arylhydrazones.
Arora, S.; Palani, V.; Hoye, T. R.
DOI: 10.1021/acs.orglett.8b03439
Org. Lett. 2018, 20, 5550–5553
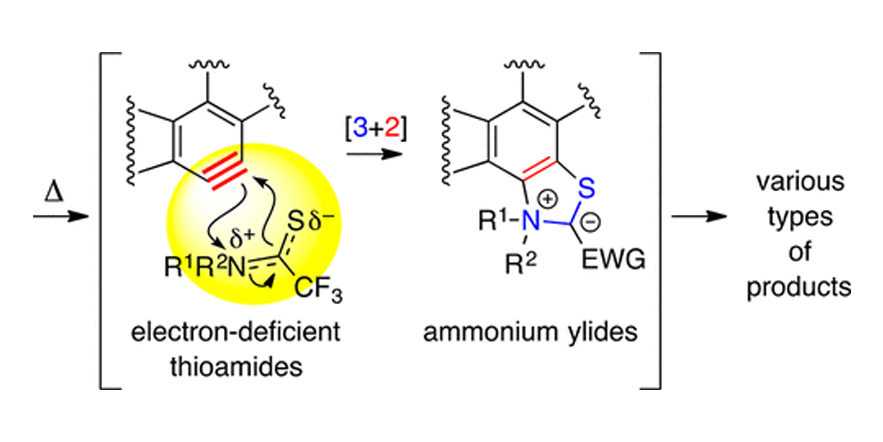
Atypical Mode of [3+2]-Cycloaddition: Pseudo-1,3-dipole Behavior in Reactions of Electron-Deficient Thioamides with Benzynes.
Zhang, J.; Page, A. C. S.; Palani, V.; Chen, J.; Hoye, T.R
DOI: 10.1021/acs.orglett.8b02133
Org. Lett. 2016, 18, 6312–6315.
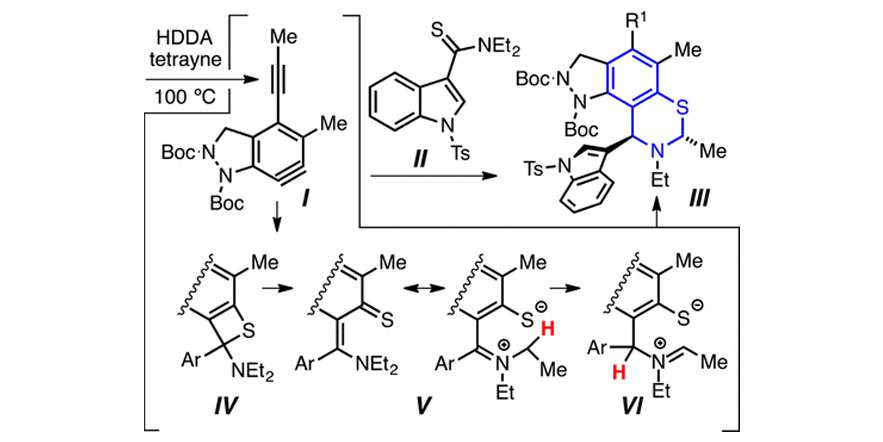
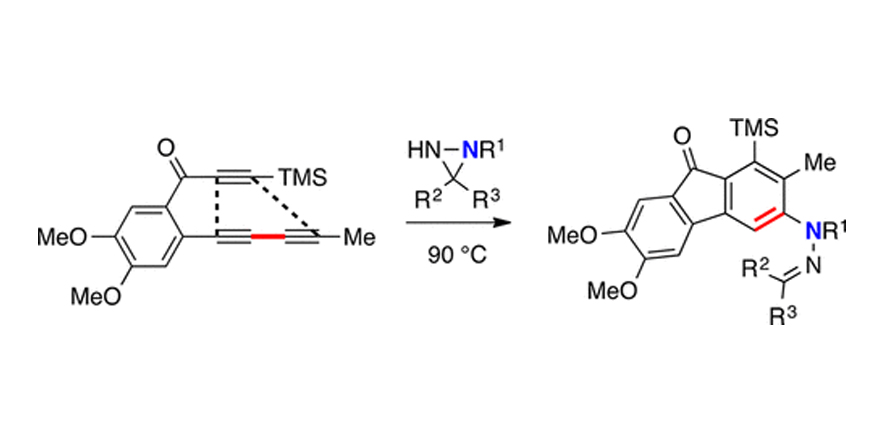
Reactions of Hexadehydro-Diels–Alder (HDDA)-Derived Benzynes with Thioamides: Synthesis of Dihydrobenzothiazino-Heterocyclics.
Palani, V.; Chen, J.; Hoye, T. R
DOI: 0.1021/acs.orglett.6b03199
J. Am. Chem. Soc. 2016, 138, 4318–4321
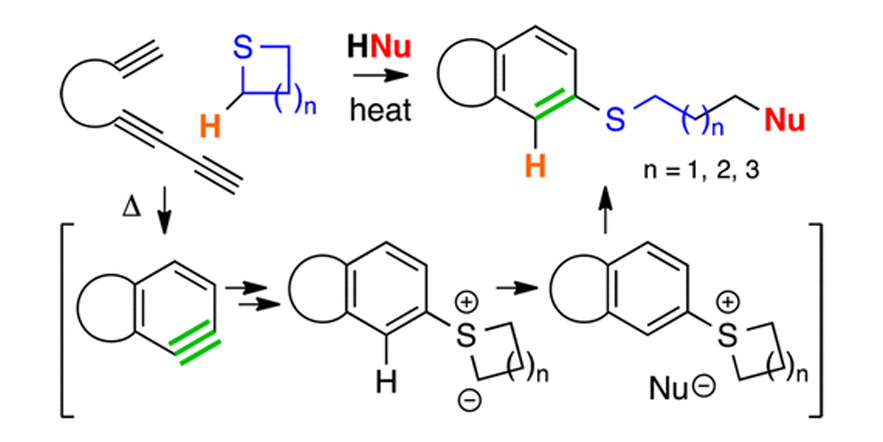
Reactions of HDDA-Derived Benzynes with Sulfides: Mechanism, Modes, and Three-Component Reactions.
Chen, J.; Palani, V.; Hoye, T. R.
DOI: 10.1021/jacs.6b01025